NSU Newsroom
SharkBytes
Horizons
This version of NSU News has been archived as of February 28, 2019. To search through archived articles, visit nova.edu/search. To access the new version of NSU News, visit news.nova.edu.
This version of SharkBytes has been archived as of February 28, 2019. To search through archived articles, visit nova.edu/search. To access the new version of SharkBytes, visit sharkbytes.nova.edu.
NSU Research Spotlight: NSU, FAU, and UTHSC Collaborate on Sea Turtle Research Project
Dr. Pallavi Patel College of Health Care Sciences
Faculty members representing Florida Atlantic University, NSU, and the University of Texas joined forces on an original research study to explore the lymphatic systems of sea turtles.
To date little is known about the anatomy and function of the lymphatics in sea turtles. These structures are difficult to image and photograph with traditional techniques and devices. Thankfully, a new imaging device is enabling real-time imaging of the lymphatics in humans and other animals. Indocyanine Green with Near Infrared Fluoroscopy (ICG-NIRF) is helping to visualize the lymphatics and lymphatic flow unlike any other imaging method. The ICG-NIRF technique is FDA cleared for investigational use.
The goal of this original research is twofold: To map the anatomy of aquatic and sea turtle lymphatics and to determine if fibropapillomatosis (FP) is related to lymphatic dysfunction resulting from environmental exposures.
The faculty members doing the research are Heather Hettrick, Ph.D., PT, CWS, CLT-LANA, CLWT, associate professor in the Department of Physical Therapy in the NSU Dr. Pallavi Patel College of Health Care Sciences; Derek Burkholder, Ph.D., research scientist at the Guy Harvey Research Institute; Sarah Milton, Ph.D., associate professor in the Department of Biological Sciences at Florida Atlantic University (FAU); and Eva Sevick-Muraca, Ph.D., distinguished chair and professor of cardiovascular research and a professor in the department of diagnostic and interventional imaging at the University of Texas Health Science Center (UTHSC).
A functioning lymphatic system is vital to overall health given the many responsibilities it performs, including the regulation of tissue homeostasis, removal of cellular debris, and immune cell trafficking (Ridner 2013). Most living creatures have a lymphatic system; however, it is not as well understood as compared to the other body systems. Although present, little is known about reptilian lymphatics.
Once turtle lymphatics are better understood, visualized, and mapped, the second part of this study will explore the poorly understood process of FP in sea turtles. This unique and potentially groundbreaking comparative pathology study will examine the pathophysiology of human papillomatosis seen in patients with lymphedema to determine if a similar etiology can help explain the development of FP in sea turtles.
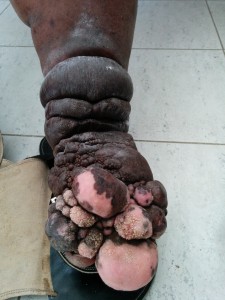
In humans, papillomatosis occurs in patients with lymphedema after frequent inflammation and episodic infection. These insults lead to more dysfunction and impairment in an already-
compromised system, leading to significant disruption in skin physiology. With FP, it has been hypothesized to be the result of a herpes infection; however, the vector that has allowed FP to spread remains unconfirmed within the sea turtle community.
This study will test the hypothesis that FP is a result of lymphatic dysfunction due to exposure to environmental toxins. FP is common in sea turtles that live in areas with high agricultural runoff and in areas of contamination affecting water quality and the turtles’ food supply. In areas of lymphatic failure, the tissues become more vulnerable to infection, inflammation, and carcinogenesis (Ruocco et al, 2007). As such, herpes seen in green sea turtles may be opportunistic, taking advantage of a vulnerable and compromised system and tissues.
This theory partially supports that the lymphatic system is responsible for the cleansing and recycling of the interstitial fluids. When overburdened with toxins, the fluids stagnate, altering fluid hemostasis. Inevitably, this leads to changes in the soft tissues, such as fibrosis and collagen proliferation. This may explain the resulting FP, and given the pathological changes, an opportunistic infection such as herpes can readily manifest.
The study will begin by utilizing ICG-NIRF to visualize and appreciate the lympathic anatomy of aquatic turtles (red-eared sliders/Trachemys scripta elegans) and the Florida softshell turtle (Apalone ferox), and then sea turtle lymphatics using green sea turtles (Chelonia mydas) with and without FP.
A three-day pilot study will be conducted at FAU involving five Florida softshell turtles and five red-eared sliders. These turtles’ lymphatics will be observed using ICG-NIRF. After injecting the turtles with ICG, the researchers will observe the uptake and flow, revealing details of the lymphatic vessels and structures. Given that ICG has a short half-life, observation will be one to two hours per turtle. Following ICG-NIRF, the turtles will be monitored for one week following ICG to observe weight, feeding habits, and signs of distress.
The subsequent study will require a permit modification to study green sea turtles (Chelonia mydas), as they are a protected species. This study will also employ ICG-NIRF, but it will involve healthy green sea turtles in addition to green sea turtles with fibropapillomatosis to distinguish if differences exist in their lymphatic systems, such as uptake, flow, congestion, or other morphological impairments. The study turtles will also be monitored for one week post-ICG to observe weight, feeding habits, and signs of distress.
Concurrently, an in-depth exploration of the current literature on both FP and human papillomatosis will be conducted to fully appreciate what is currently known about both conditions. Collaboration with marine biology experts and lymphology experts will explore the histology, genetic, and even molecular presentations of these conditions. The expected outcomes are to better appreciate and understand sea turtle lymphatics, and to determine if, in part, the pathophysiology of human lymphatic failure can partially explain the process of FP in sea turtles, possibly leading to interventions for prevention and management.
References
Ridner S. Pathophysiology of lymphedema. Semin Oncol Nurs. 2013; 29:4-11.
Ruocco V, Ruocco E, Brunetti G, et al. Opportunistic localization of skin lesions on vulnerable areas. Clinics in Dermatology. 2011; 29:483-488.